How do clinicians know if they are treating patients too much, too little or just right? As continuing professional education (CPE) professionals, we focus on providing access to education that helps clinicians balance evidence-based science with the guidance of experts when the evidence is incomplete. The safety and efficacy of many therapies have not been well studied in certain populations that do not meet eligibility criteria for clinical trial participation due to patient- or disease-specific factors.
Examples of “underserved populations” include individuals with multiple comorbidities, who may have compromised immune function or who are elderly. Appropriate use of therapies for these individuals is surrounded by uncertainty and the unclear applicability of clinical trial findings to routine practice. As a result of the evidence shortage, clinicians must use their best judgement to manage patients who fall in these categories and, in some cases, may not offer treatments to patients who might benefit from them.1-4
Eligibility for randomized clinical trials is typically limited to individuals who are most likely to tolerate systemic treatment and less likely to experience complications or adverse effects. Clinical trial participants are usually those with high likelihood of good outcomes. Elderly patients is one population underrepresented in clinical trials. For instance, an estimated 24% of oncology trial participants are 70 years of age or older, but this group represents 42% of the cancer population.5 Thus, the effectiveness and safety of newer agents in special populations is not well understood. Moreover, these restrictions exclude the types of patients who are managed in routine clinical practice, posing a challenge for clinicians to extrapolate clinical trial evidence to their patients in real-life practice. Rawad Elias, MD, oncologist at the Hartford HealthCare Cancer Institute in Connecticut, said, “We don’t really understand the clinical profile of these drugs in the real-world population.” 6
Faced with the lack of data and resultant uncertainty, clinicians may withhold therapies from special populations who mostly do not meet trial inclusion criteria. Authors of a recent narrative review wrote: “In clinical practice, elderly patients are treated mostly with palliative rather than curative intent.” 7
Data from continuing professional development (CPD) activities that include tools, like chatbots, can provide insights on the search patterns of clinicians and how they go about questioning data to make informed decisions about treatment approaches when minimal evidence is available. For example, oncologists have different questions and varying perspectives than their otolaryngologist colleagues as seen in Figure 1 below.
Figure 1: Questions by Specialist Type in AI-driven @sk|ChatBot 8
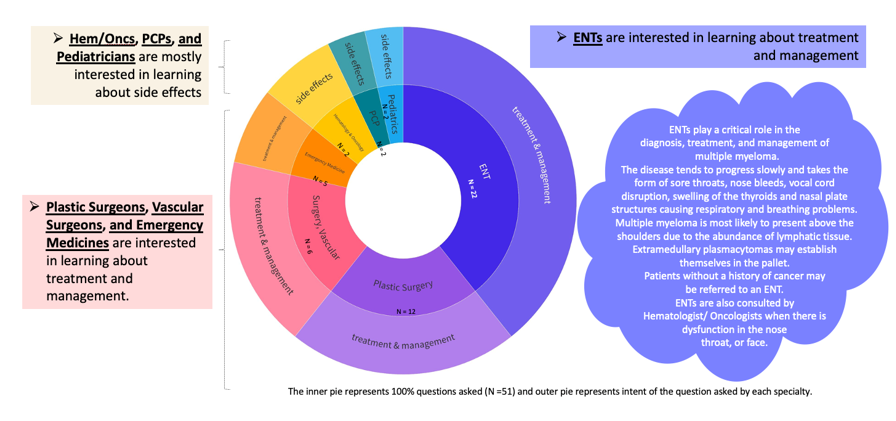
Chatbots can be helpful when considering the needs of underrepresented populations as clinicians consider factors for personalizing care. These tools can highlight insights about clinician queries while developing treatment plans. Questions (see example of types of questions, Figure 2, below) can be tracked by clinical specialty to provide a glimpse into challenges faced by multi-disciplinary healthcare professionals (HCPs) who may have a different focus for patient care.
Figure 2: Sample of Verbatim Learner Questions Entered Into AI-driven @sk|ChatBot 8
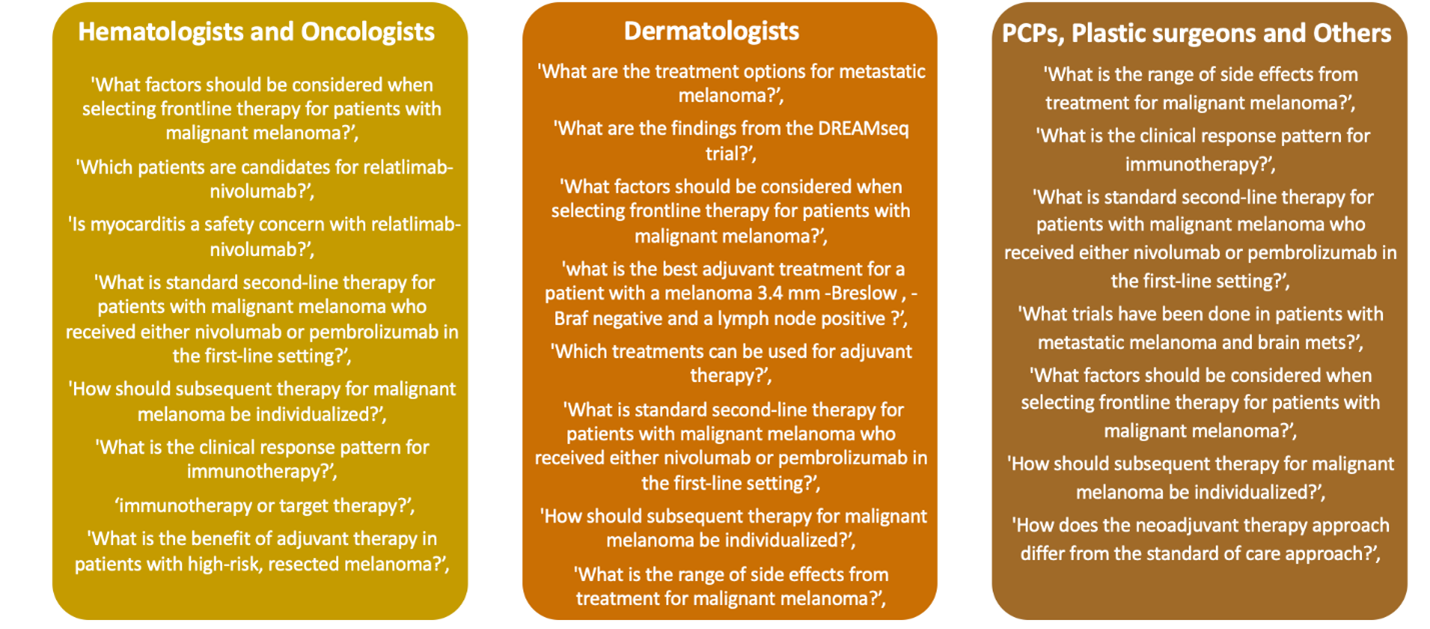
As medicine continues to advance, clinicians will face challenges with incorporating new data into their personalized care plans for individual patients (so-called Goldilocks treatments). Tools that provide insights into these complex decisions can help make CPD activities more impactful across disease areas.
Projects In Knowledge Powered by Kaplan will continue exploring these challenges of therapeutic choice in settings of limited evidence with a discussion among experts titled: “Clinical Conversations: Actionable Strategies for Immunotherapy in Underrepresented Trial Populations.” The on-demand enduring activity will be available starting in February 2024. Outcomes from the activity will be shared in a future Almanac article.
 Alana Brody, MBA, CHCP, is executive director and vice president, strategic educational design, for Projects In Knowledge Powered By Kaplan. She has been involved in the healthcare field for over two decades supporting nonprofit organizations such as the National Comprehensive Cancer Network (NCCN), New England Journal of Medicine (NEJM), as well as for-profit organizations including Kaplan. She has earned the Alliance’s Distinguished Member designation and is currently a member of the Alliance Board of Directors.
Alana Brody, MBA, CHCP, is executive director and vice president, strategic educational design, for Projects In Knowledge Powered By Kaplan. She has been involved in the healthcare field for over two decades supporting nonprofit organizations such as the National Comprehensive Cancer Network (NCCN), New England Journal of Medicine (NEJM), as well as for-profit organizations including Kaplan. She has earned the Alliance’s Distinguished Member designation and is currently a member of the Alliance Board of Directors.
 Susan Miller, Ph.D., has been the director of grants strategy at Projects In Knowledge Powered by Kaplan since 2018. Dr. Miller has extensive experience as a medical writer, having written medical education content across a wide range of therapeutic areas and countless needs assessments for grant proposals, with a proven record of funding acquisition. Prior to Projects In Knowledge, she was founder and principal at Green River Medical Writing and had spent over 12 years in diverse academic research environments, examining the functions and regulatory pathways of oncogenic and tumor suppressive proteins involved in cancer. Dr. Miller received her B.S. in biology, magna cum laude, from the University of Utah and her Ph.D. in molecular biology from the University of Texas Graduate School of Biomedical Sciences (GSBS), now MD Anderson UTHealth GSBS. Her prior positions include post-doctoral fellowships at Harvard University in which she was awarded research grants from the American Cancer Society and the Medical Foundation. She was awarded a pre-doctoral fellowship from the American Legion Auxiliary, University of Texas, and was winner of the alumni award for graduate student research.
Susan Miller, Ph.D., has been the director of grants strategy at Projects In Knowledge Powered by Kaplan since 2018. Dr. Miller has extensive experience as a medical writer, having written medical education content across a wide range of therapeutic areas and countless needs assessments for grant proposals, with a proven record of funding acquisition. Prior to Projects In Knowledge, she was founder and principal at Green River Medical Writing and had spent over 12 years in diverse academic research environments, examining the functions and regulatory pathways of oncogenic and tumor suppressive proteins involved in cancer. Dr. Miller received her B.S. in biology, magna cum laude, from the University of Utah and her Ph.D. in molecular biology from the University of Texas Graduate School of Biomedical Sciences (GSBS), now MD Anderson UTHealth GSBS. Her prior positions include post-doctoral fellowships at Harvard University in which she was awarded research grants from the American Cancer Society and the Medical Foundation. She was awarded a pre-doctoral fellowship from the American Legion Auxiliary, University of Texas, and was winner of the alumni award for graduate student research.
References:
1. Zhang D, Tailor TD, Kim C, Atkins MB, Braithwaite D, Akinyemiju T. Immunotherapy utilization among patients with metastatic NSCLC: Impact of comorbidities. J Immunother. 2021;44(5):198-203.
2. Raphael J, Richard L, Lam M, et al. Utilization of immunotherapy in patients with cancer treated in routine care settings: a population-based study using health administrative data. Oncologist. 2022;27(8):675-684.
3. Jain V, Hwang WT, Venigalla S, et al. Association of age with efficacy of immunotherapy in metastatic melanoma. Oncologist. 2020;25(2):e381-e385.
4. Freeman SC, Satish M, Walters RW. Comorbidity burden on receipt of adjuvant immunotherapy and survival in patients with stage III melanoma: an analysis of the National Cancer Database. Int J Dermatol. 2020;59(11):1381-1390.
5. Sedrak MS, Freedman RA, Cohen HJ, et al. Cancer and Aging Research Group (CARG). Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J Clin. 2021;71:78-92.
6. Yasinski E. Immunotherapy in the elderly. Cancer Today. July 16, 2019. Accessed at: https://www.cancertodaymag.org/cancer-talk/immunotherapy-in-the-elderly/.
7. Montrone M, Rosati G, Longo V, et al. Immunotherapy in elderly patients affected by non-small cell lung cancer: a narrative review. J Clin Med. 2023;12(5):1833.
8. Data on File: Projects In Knowledge Powered By Kaplan. 2023.