Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn's disease (CD), is a chronic and recurrent inflammatory disease of the intestinal tract that currently affects as many as three million Americans1. The disruptive nature of the disease course, particularly when suboptimally managed, significantly reduces the patient’s quality of life and increases disease-related complications, leading to a significant impact on patients’ psychosocial, financial and physical well-being. Early diagnosis and effective treatment may reduce or prevent exacerbation and modify disease course. 2-8
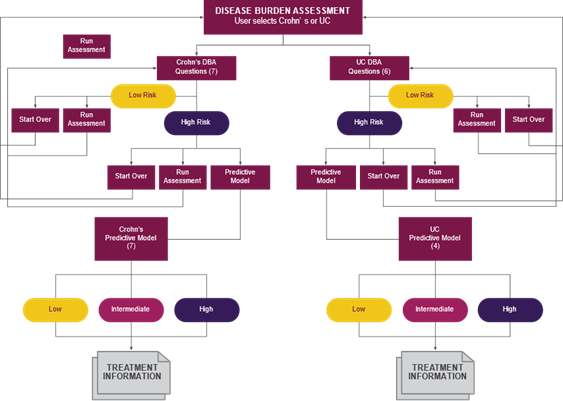
Before the development of the IBD Clinical Decision Support Tool (CDST), available tools were effective but limited in scope: some predicted complication risk over time while others helped clinicians navigate treatment algorithms. None predicted a patient’s response to treatment with a biologic, delaying the initiation of effective personalized treatment. The U.S. Health Outcomes Consortium headed by Parambir Dulai, MD, developed the IBD CDST to address the need for a validated, drug-specific, user-friendly, prediction tool. This free tool, which can be accessed on any internet-connected device, allows clinicians to assess risk and optimize treatment selection in real time, facilitating the early implementation of effective treatment strategies (fig. 1) 9.
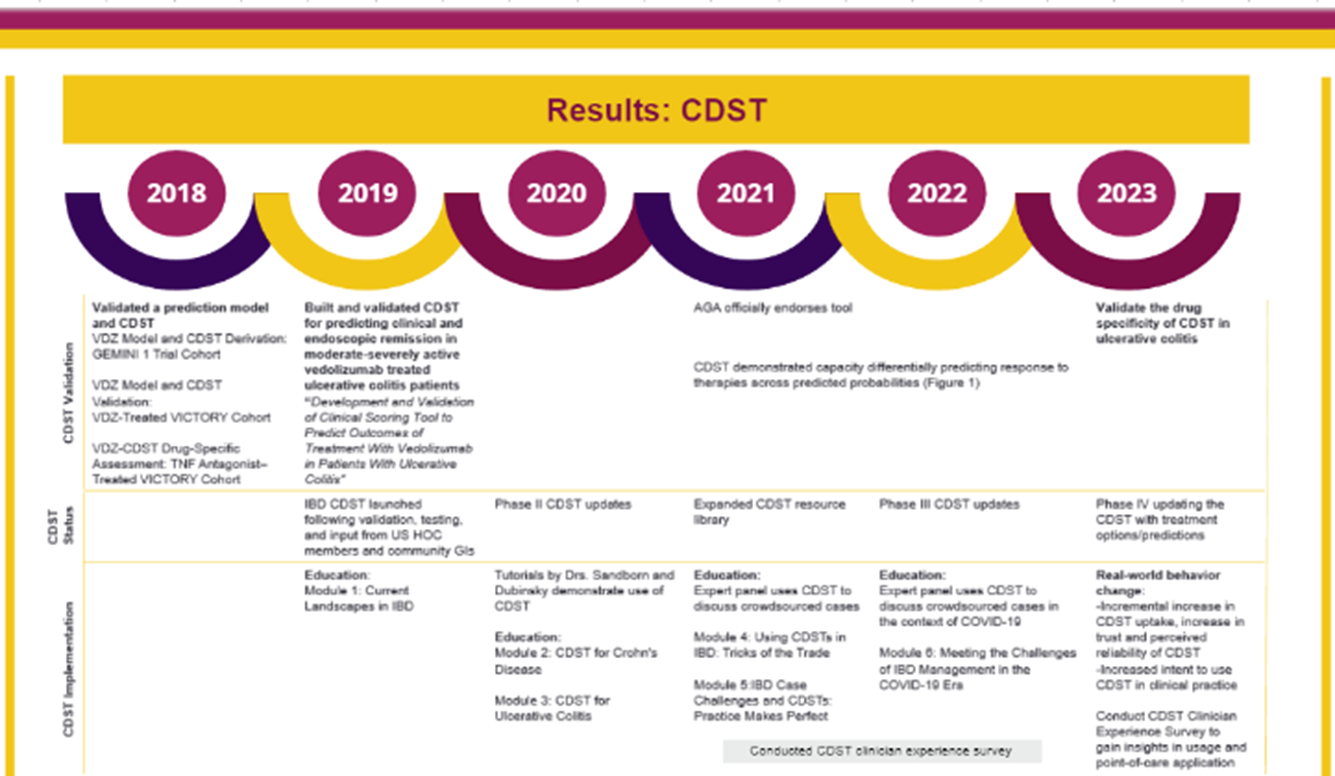
The U.S. Health Outcomes Consortium — in partnership with RMEI and supported by independent medical education grants from Takeda — developed a multiphasic educational strategy to expedite integration of the tool into clinical settings (fig. 2). The education was cumulative and linear, bringing the learner population from awareness to implementation over the course of four years. This strategy ensured that clinicians received dynamic and relevant support while mitigating the burnout effect of being “always online” 10,11.
Patient level data from the VARSITY (n=207 vedolizumab; n=212 adalimumab) and OCTAVE (n=897 tofacitinib) trials were obtained through an open access platform (Vivli). Baseline demographics for all patients (endoscopic activity, TNF-antagonist exposure, disease duration, albumin) were used to calculate probability of response (low, intermediate, high) and assessments were made for differential prediction of endoscopic remission (Mayo endoscopic sub-score 0; blinded central reads) for the three drugs among the three predicted probability of response sub-groups. The CDST predictive models were validated through multiple studies 12-15.
The curricula which accompanied the release of the IBD CDST assessed learner’s changes in their attitudes, opinions, proficiency and confidence. Curriculum content, designed for a gastroenterology specialized audience, included detailed explanation of the model, how the model was validated, how to use the tool, and how to communicate the findings effectively to patients and colleagues. Changes in proficiency were measured by calculating the change ratio from pre- to post-test on objective aggregate metrics comprised of individual multiple-choice or Likert-scale questions.
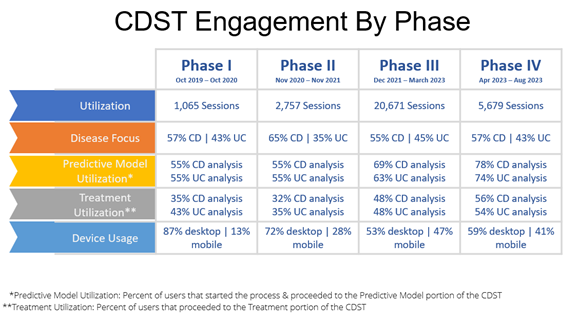
Across all educational phases, a total of 12,787 participants were reached. Two-thousand and seventy-six clinicians participated in the education, and 1,593 learners completed the content and testing then pivoted their 243% increase in demonstrated mastery (answering a question correctly and reporting a high degree of confidence in their response) into increased IBD CDST usage (fig. 3). Learners reported intent to implement the tool translated directly to clinical behavior change, specifically progressive increase in the frequency of CDST usage between October 2019 and August 2023. Thirty-thousand and one-hundred seventy-two patient scenarios have been input into the IBD CDST to date.
Notably, engagement in the education and subsequent implementation of the IBD CDST extended beyond gastroenterologists (GIs). Sixty-two percent of GI learners switched from “will not use CDST” to “will use CDST” by post-test resulting in 100% of responding GIs intending to integrate the tool into their practice. Other members of the multi-disciplinary care team — 75% of primary care practitioners (PCP) and 80% of all learners — also reported they will use the IBD CDST in ways that align with their role in the care of patients with IBD (fig. 4).
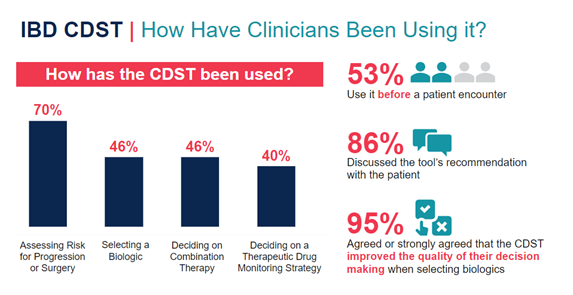
Open-ended feedback from learners who went on to use the CDST in practice included reports that the tool improved: their selection of biologics, their decision-making and, subsequently, their patient’s outcomes. These learners also reported that they would incorporate the CDST into patient communication and share the CDST with their colleagues.
The use of an iterative, multiphasic, educational strategy to facilitate the learning pathway from awareness to integration was successful. Learners became familiar with the tool, developed confidence in the tool, learned how to use the tool, and were provided strategies on how to integrate the tool and its findings into the flow of their practice. As a direct result of the supportive successive curricula, progressive engagement with the tool was observed, culminating in organic peer-to-peer dissemination and endorsement. Most IBD CDST sessions involved the inputting of patient data to assess risk and/or develop a treatment response model, indicating that these sessions were part of users’ clinical practice. Beyond the demonstrable uptick in use, substantial increases in learner confidence were also observed with users going as far as to credit their perceived improvement in patient outcomes to their use of the IBD CDST. Taken together, these findings reflect the efficacy of education as a vehicle for rapid integration of point-of-care tools into clinical practice.
 Elizabeth Johnson, MA, is the director of outcomes, analytics and research at RMEI. She holds a bachelor’s degree in psychology and a master’s degree in forensic psychology. Before coming to med ed, she was a senior clinical case manager for the forensic population which informs her special interest in patient centricity. Elizabeth has worked in medical education as an analyst and researcher for eight years.
Elizabeth Johnson, MA, is the director of outcomes, analytics and research at RMEI. She holds a bachelor’s degree in psychology and a master’s degree in forensic psychology. Before coming to med ed, she was a senior clinical case manager for the forensic population which informs her special interest in patient centricity. Elizabeth has worked in medical education as an analyst and researcher for eight years.
 Nicolette Theriault, MPH, is the outcomes and analytics manager at RMEI and has been with RMEI since 2020. Her diverse background includes health programming, research, project management, writing and publications. Nicolette earned her BA in public relations and fine art from Quinnipiac University and her master’s in internal public health from the University of Liverpool in the U.K.
Nicolette Theriault, MPH, is the outcomes and analytics manager at RMEI and has been with RMEI since 2020. Her diverse background includes health programming, research, project management, writing and publications. Nicolette earned her BA in public relations and fine art from Quinnipiac University and her master’s in internal public health from the University of Liverpool in the U.K.
 Lobna Eldasher, PharmD, is a senior medical director at RMEI Medical Education. She earned her Doctor of Pharmacy degree from the Ernest Mario School of Pharmacy at Rutgers University where she conducted research in public and environmental health. She has worked in medical writing and continuing healthcare professional education for 10 years, with a focus on inflammation and inflammatory diseases.
Lobna Eldasher, PharmD, is a senior medical director at RMEI Medical Education. She earned her Doctor of Pharmacy degree from the Ernest Mario School of Pharmacy at Rutgers University where she conducted research in public and environmental health. She has worked in medical writing and continuing healthcare professional education for 10 years, with a focus on inflammation and inflammatory diseases.
References:
- Prevalence of IBD. 2022. National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. Prevalence of IBD | CDC
- Feuerstein JD, et al. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology. 2020 Apr;158(5):1450-1461. doi: 10.1053/j.gastro.2020.01.006.
- Feuerstein JD, et al. AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn's Disease. Gastroenterology. 2021 Jun;160(7):2496-2508. doi: 10.1053/j.gastro.2021.04.022
- Siegel CA, Whitman CB, Spiegel BMR, et al. Development of an index to define overall disease severity in IBD. Gut 2018;67:244-254.
- Siegel CA, et al. Real-time tool to display the predicted disease course and treatment response for children with Crohn's disease. Inflamm Bowel Dis. 2011;17(1):30-38
- Guizzetti L, et al. Development of Clinical Prediction Models for Surgery and Complications in Crohn's Disease. J Crohns Colitis. 2018;12(2):167-177.
- Dulai PS, et al. J Crohns Colitis. 2019;13(S1):S388.
- Sandborn WJ. Crohn's disease evaluation and treatment: clinical decision tool. Gastroenterology. 2014;147(3):702-705.
- Seigel CA, et al. Treatment Pathways Leading to Biologic Therapies for Ulcerative Colitis and Crohn's Disease in the United States. Clin Transl Gastroenterol. 2020 Feb;11(2):e00128.
- Kemp. S. DIGITAL 2022: TIME SPENT USING CONNECTED TECH CONTINUES TO RISE. Digital 2022: Time Spent Using Connected Tech Continues to Rise — DataReportal – Global Digital Insights
- IPSOS. 2021. Seven in ten Americans say they have been spending more time online this year than ever before. Seven in ten Americans say they have been spending more time online this year than ever before | Ipsos
- Dulai PS, et al. Inflamm Bowel Dis. 2022 Oct 3;28(10):1555-1564. Decision Support Tool Identifies Ulcerative Colitis Patients Most Likely to Achieve Remission With Vedolizumab vs Adalimumab - PubMed (nih.gov)
- Dulai PS, et al. Development and Validation of Clinical Scoring Tool to Predict Outcomes of Treatment With Vedolizumab in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020 Dec;18(13):2952-2961.e8.
- Dulai PS, et al. Development and Validation of a Scoring System to Predict Outcomes of Vedolizumab Treatment in Patients With Crohn's Disease. Gastroenterology. 2018 Sep;155(3):687-695.e10.
- Dulai PS, et al. Clinical Prediction Model and Decision Support Tool for Ustekinumab in Crohn's Disease. Am J Gastroenterol. October 2019;114:S373. Abstract #637.