Elena K. Joerns, PI, is a Rheumatology Research Fellow.
Institutional Affiliation: University of Texas Southwestern Medical Center, Dallas
To support initiatives to improve public health through the education of healthcare providers and patients, the Alliance and Pfizer partnered to create opportunities focused on increasing immunization rates of adults who are at increased risk for pneumococcal disease. Read an introduction to this series here.
Project Description
Our goal was to implement a nurse-driven workflow to improve the pneumococcal vaccination rate by 10% over a 16-month period in immunosuppressed patients aged 19–64 years old, followed at the UT Southwestern adult rheumatology clinic.
Eligible adults aged 19–64 years old in the electronic medical record (EMR) were identified using an automated search protocol based on age and pre-set immunosuppressive medications list. We calculated baseline vaccination rate from Jan. 1–Dec. 31, 2019. We placed CDC educational brochures in waiting and exam rooms in our clinic to increase patient awareness and acceptance of the pneumococcal vaccination.
We created a pneumococcal vaccination protocol based on Centers for Disease Control (CDC) guidelines1 and converted it to a university-approved standing medical order (SMO) for the clinic staff to use. A nurse-driven workflow was developed for vaccination implementation in the clinic. We held multiple educational sessions for the staff to review the protocol and workflow, and re-training was performed as needed. We relied on monthly clinic staff feedback to implement Plan-Do-Study-Act (PDSA) cycles to improve the workflow and monthly vaccination rates. In addition, we educated the providers on the ACIP guidelines for pneumococcal vaccination1. As a reflection of the importance of the project, our group has adopted pneumococcal vaccination rates as an incentive goal for 2021.
Post-intervention pneumococcal vaccination rates were calculated monthly. The start date for the intervention phase of project was Jan. 1, 2020. We obtained post-intervention vaccination data until March 2020 when clinics closed due to the COVID-19 pandemic, then continued monthly data collection after the clinics reopened in June 2020. Documentation of vaccination refusal by patients was monitored monthly. We assessed the percentage of vaccinated patients in our clinic and compared this with baseline rates.
Accomplishments
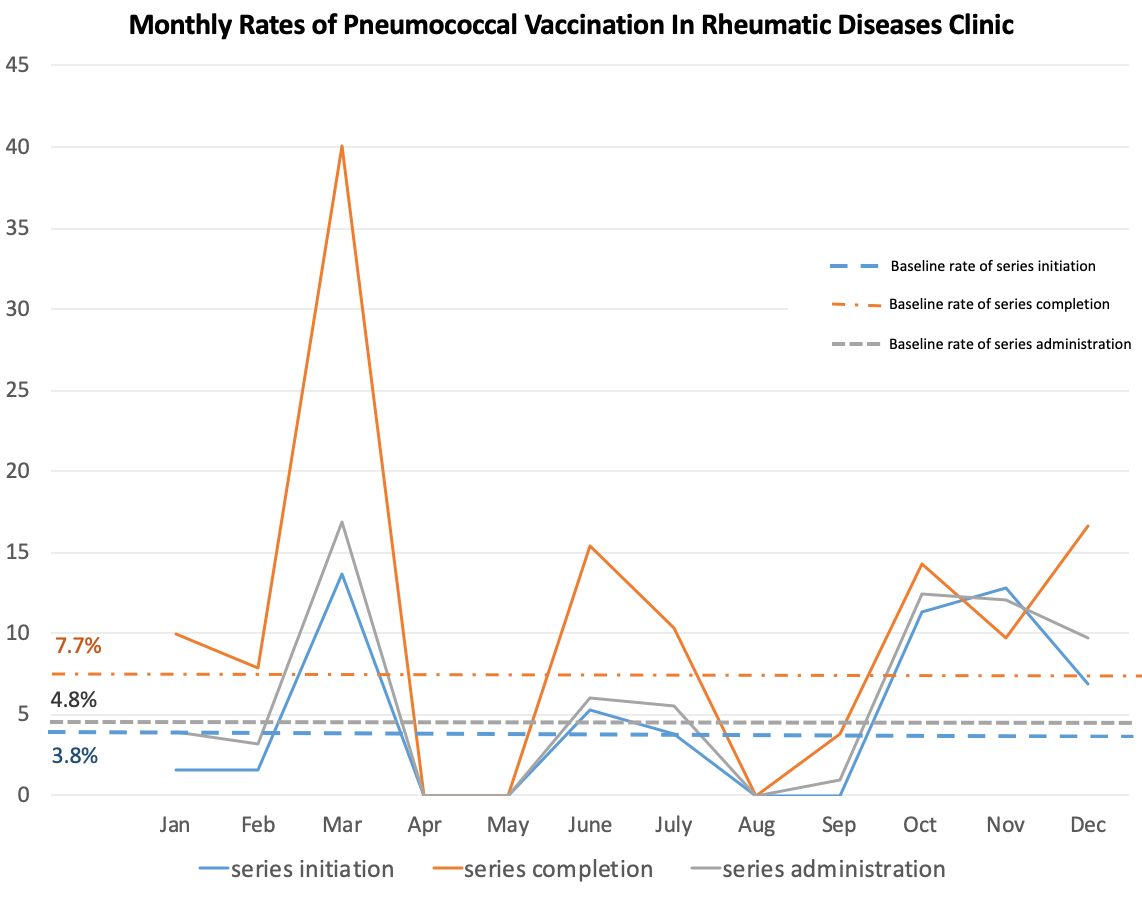
The baseline rate for full immunization of immunosuppressed rheumatology patients aged 19–64 years in 2019 was 6.6%. The baseline average monthly vaccination series initiation rate in 2019 was 3.8%. From January–March 2020, we achieved an 11.4% vaccination rate of previously unvaccinated patients.
Due to COVID-19, from April–September 2020, only 50% of the staff were in the clinic at a time. We achieved a 0% vaccination initiation rate and a 3.8% series completion rate in September 2020 after an initial 5.3% series initiation rate and a 15.4% series completion rate in June 2020. In October 2020, the clinic was fully staffed. In the period from October–December 2020, we achieved up to a 12.8% vaccination initiation rate and a 16.7% series completion rate. (See Figure 1).
The project was presented in a poster format during the Annual Convergence meeting of American College of Rheumatology in November 20202.
Challenges
In March 2020, our clinics closed due to the COVID-19 pandemic. The clinics reopened in June 2020 at about 40% capacity, which has since been fluctuating. After reopening, only 50% of the staff was on-site at a time, and quality efforts were focused on COVID-19 infection reduction-focused protocols and work-flows. With full the full reopening of the clinic in October 2020 and 100% of staff returned to the in-person clinic workflow, we were once again able to engage the staff in our nurse-driven protocol.
Recent additional challenges include the constraints of the COVID-19 vaccination. The COVID-19 mRNA vaccine should not be administered within 14 days of another vaccination3. As many of our patients are immunosuppressed, COVID-19 vaccination is a priority and we are recommending delaying pneumococcal vaccination in those patients scheduled for the COVID-19 vaccine4. This strategy will decrease the monthly pneumococcal vaccination rates until most of our patients are fully immunized against COVID-19.
Lessons Learned
Our study suggests that a multimodal intervention using a nurse-driven workflow utilizing a SMO is a useful tool in improving pneumococcal vaccination rates in a rheumatology clinic. We have learned the importance of obtaining and providing regular data-related feedback with the staff and sharing the improvements. In addition, our project demonstrated that being able to adapt quickly to the changing environments of the clinic can help in improving the workflow.
Figure 1: Monthly Pneumococcal Vaccination Rates, January–December 2020
Vertical axis: Percentage vaccinated in a given month
References
- Morbidity and Mortality Weekly Report. “Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine
for Adults with Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP).” MMWR. Oct 2012. 61 (40).
- Joerns E, Pokala N, Reisch JS, Wang D, Arasaratnam R, Bermas BL, Bajaj P. Improving Pbneumococcal Vacination Rates Among Immunosuppressed Adults in an Academic Rheumatology Clinic Utilizing a Nurse Driven Protocol [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/improving-pneumococcal-vaccination-rates-among-immunosuppressed-adults-in-an-academic-rheumatology-clinic-utilizing-a-nurse-driven-protocol/.
- gov. Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines Currently Authorized in the United States. National Center for Immunization and Respiratory Diseases. Jan 2021.
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ Updated Interim Recommendation for Allocation of COVID-19 Vaccine – United States, Dec 2020. MMWR Morb Mortal Wkly Rep 2021. 69:1657-1660.